|
Dr. Richard Murphy Director of Research for the Alltech European Bioscience Center considered the modulation of antimicrobial-resistance in a presentation in the poultry session at the recent Alltech ONE Virtual Experience. Murphy stressed that the microbiome is involved in multiple functions including suppression of pathogens, modulating the immune system and influencing intestinal morphology. The intestinal microbiome may be considered as an additional organ enhancing digestion and contributing to health and productivity.
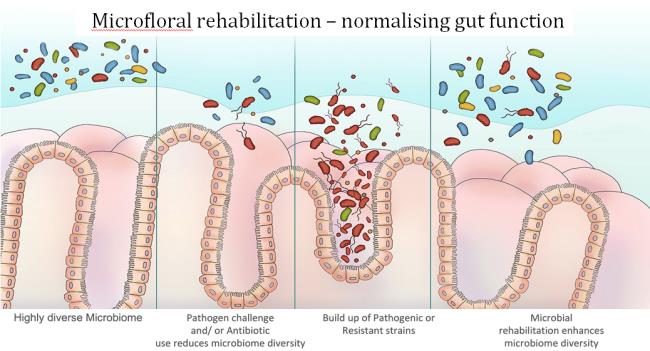
Numerous factors influence the composition of the microbiome including feed ingredients, and in the case of poultry, ingestion of litter, pH of water and medication including antibiotics. It is generally conceded that a functional and beneficial microbiome is characterized by diversity. Unfortunately antibiotics severely reduce diversity and thereby depress the beneficial effects of a normal balanced flora. Reducing diversity permits the proliferation of pathogens resulting in deleterious changes within the intestinal tract. The use of antibiotics for growth promotion or therapy is associated with excretion of non-absorbed antibiotic and metabolites into the environment. It is estimated that with respect to some antibiotics, 90 percent may be excreted in urine and 75 percent in feces. Injudicious use of antibiotics on a specific farm will inevitably result in the emergence of antibiotic resistance. This will contribute to dissemination of antibiotic-resistant organisms through infected soil, water ansd products. Studies conducted in Europe and especially in Denmark show that antibiotic resistance can persist in livestock on a specific farm for prolonged periods.
|

Dr. Richard Murphy |
 Murphy suggests that strategies to rehabilitate the intestinal microbiome can reverse the deleterious effect of administration of growth-stimulating antibiotics and antibiotic therapy. Prebiotics that demonstrate efficacy can reintroduce diversity to the intestinal microbiome with beneficial effects on health and productivity. There are number of feed additives that can influence the microbiome including prebiotics, active live probiotics, enzymes that assist in degradation of nutrients, botanicals and short-chain fatty acids.
Murphy suggests that strategies to rehabilitate the intestinal microbiome can reverse the deleterious effect of administration of growth-stimulating antibiotics and antibiotic therapy. Prebiotics that demonstrate efficacy can reintroduce diversity to the intestinal microbiome with beneficial effects on health and productivity. There are number of feed additives that can influence the microbiome including prebiotics, active live probiotics, enzymes that assist in degradation of nutrients, botanicals and short-chain fatty acids.
First generation prebiotics comprising extracts from the cell wall of the common yeast (Saccharomyces cerevisiae) including mannanoligosaccharides, were introduced over 30 years ago and marketed as Bio-Mos™. Subsequent research demonstrated that an extract from MOS termed the mannan-rich fraction (MRF) demonstrated higher activity and induced a more pronounced beneficial response in the microbiome. Both MOS and MRF prebiotics have the ability to adsorb pathogenic bacteria with Type-1 fimbriae including Salmonella and E. coli. By modulating the microbiome, intestinal integrity may be improved together with stimulation of the immune response by the intestine.
Murphy stressed that the greatest benefit in broilers is derived from administration of the MRF prebiotic early in the growing cycle to establish a beneficial flora that persists through to depletion.
Murphy noted that in addition to diversity, as a result of including MRF in feed, the microbiome became more balanced with a move away from a Gram-positive over-predominance. A balanced microbiome is generally more resistant to colonization by pathogens than a microflora that is biased in favor of a particular bacterial grouping. In flocks with a balanced and diverse microbiome, positive effects on intestinal morphology are evident from the duodenum extending through to the cecum.
Recently attention on rehabilitation of the microbiome in monogastric livestock has focused on antimicrobial resistance. There are multiple classes and types of antibiotics many of which were previously administered to poultry, resulting in the emergence of drug-resistant pathogens. Bacteria can extend antibiotic resistance either through exchange of plasmids, chromosomal transfer or indirectly by phages. Newer research has identified the presence of more than one-hundred gene markers in the broiler cecum conferring resistance to multiple individual antibiotics. This overall group of resistance genes is referred to as the resistome and can be further categorized into the so-called core and accessory groupings. Resistance in flocks can either be represented by widespread dissemination within the flock referred to as core resistance. In contrast when only a few birds in a flock carry particular antibiotic-resistant genes a state of accessory resistance pertains.
Inclusion of effective prebiotics in diets suppresses the intensity of core resistance. This has implications for public health as it enhances the ability of the microbiome to suppress antibiotic resistant pathogens. Studies conducted in Brazil over extended periods demonstrated a significant and progressive decrease in the recovery of antibiotic-resistant bacteria from the intestinal tract. A broiler complex processing 1.5 millions per week over a two-year period experienced a four-fold decrease in recovery of drug-resistant pathogens of human health significance attesting to the value of a diverse microbiome.