 Amid calls for the food-related responsibilities of the U.S. Food and Drug Administration to be transferred to a new agency, there are questions over the extent of supervision exercised by the FDA over foreign pharmaceutical plants. The Economist recently highlighted the problems of Marion Biotech and Maiden Pharma that were allegedly responsible for mortality in children in Gambia and Uzbekistan as a result of cough syrup manufactured with a toxic ingredient. Both Marion and Maiden are small, privately-held companies that are presumably under the jurisdiction of Pharmexcil, a trade group in India that operates with government recognition.
Amid calls for the food-related responsibilities of the U.S. Food and Drug Administration to be transferred to a new agency, there are questions over the extent of supervision exercised by the FDA over foreign pharmaceutical plants. The Economist recently highlighted the problems of Marion Biotech and Maiden Pharma that were allegedly responsible for mortality in children in Gambia and Uzbekistan as a result of cough syrup manufactured with a toxic ingredient. Both Marion and Maiden are small, privately-held companies that are presumably under the jurisdiction of Pharmexcil, a trade group in India that operates with government recognition.
India has approximately 10,000 pharmaceutical plants operated by 3,000 companies producing drugs valued at $50 billion, annually. Approximately 40 percent of America’s generic drugs are imported from India.
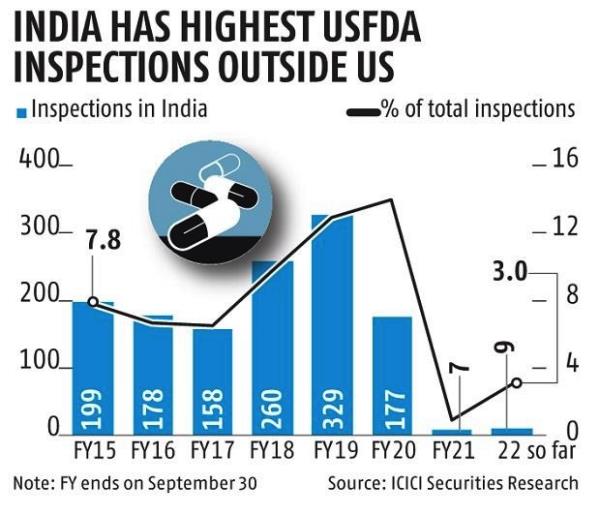
In 2022, the Food and Drug Administration sanctioned four large companies in India, blocking specific shipments. It is widely acknowledged that drug manufacturers in India operate with a strong profit motive and minimal government supervision. Domestic whistleblowers are ignored or threatened with government sanctions for disparaging the image of the Indian pharmaceutical industry and impacting exports. In the absence of a functional legal system, civil litigation is ineffective.
Given the frequency of adverse reactions and evidence of contaminated and impotent drugs, the safety of generics imported from India is questioned. We have only the FDA standing between unscrupulous manufacturers and consumers in the U. S.
Two questions arise. Is the FDA maintaining appropriate surveillance over drugs manufactured in India and other Asian nations? If not, do they require additional resources to conduct in-company inspections that, it is understood, occur at infrequent intervals? The second question is why the U. S. is dependent on nations, such as China and India, for generic drugs? Why can these products not be manufactured locally, given that the pharmaceutical industry is not labor intensive but is reliant on trained technicians and quality control personnel?
Separating food from the FDA will allow the Agency to concentrate on drugs and medical devices for which it apparently has an adequate functional organizational structure and staffing.
Who confirmed my daily generic was safe? |