Russian COVID-19 Vaccine Data is Promising
|
11/27/2020 |
|
Following initial skepticism over the speed at which the Gameleya Center for Epidemiology and Microbiology developed the "Sputnik V" vaccine, new data released in the form of a press release, claims 92 percent efficacy in a trial involving 18,000 subjects. The initial report in October based on a group of twenty recipients was greeted with scientific incredulity in early November but results of the larger trial should soon be published in a peer-reviewed journal.
The Russian vaccine uses simian adenovirus vectors to deliver a gene coding for the spike protein of SARS-CoV-2. The first dose comprises an Ad26 vector followed by a booster twenty-one days later with an Ad5 vector. The purpose of using two separate vectors is to prevent antibody response from the first vaccination interfering with the booster.
The AstraZeneca-Oxford University vaccine uses a single simian adenovirus Ad5 as a vector. This vaccine had an average efficacy of 70 percent but when, inadvertently, a subgroup of volunteers received a half-dose of the first vaccine followed by a full dose of the second vaccine, 90 percent protection was apparently achieved. Although no explanation for the discrepancy has been provided by AstraZeneca it is possible that developers in Russia anticipated the problem of antibody resistance to the booster dose, hence selection of an alternative adenovirus vector.
|
Russian Sputnik -5 two-dose COVID vaccine |
|
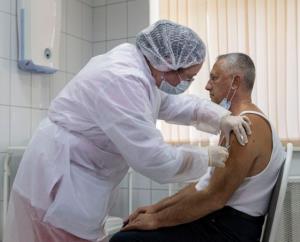
The claim of 90 percent protection for the Sputnik V vaccine is based on thirty-nine cases with eight among the vaccinated group and thirty-one in the volunteers in the control group receiving the placebo. This group comprised 25 percent of the approximately 18,000 in the trial. A subsequent analysis will be carried out when seventy-eight confirmed COVID cases are diagnosed. The trial will soon be expanded by recruitment of up to 40,000 participants.
The adenovirus-vectored vaccines are stable at refrigeration temperature and are relatively inexpensive to produce and distribute. Russia has already capitalized on their product, offering it to developing nations as a goodwill gesture. Partners of the Gamaleya Center will produce the vaccine in India, South Korea and Brazil under a license that will result in a cost of under $10 per dose. The combined output of nations producing the vaccine could attain one billion doses through 2021.
|

|
|
|