 EGG-NEWS has reported previously on eye infections arising from the use of contaminated eye drops manufactured by Global Pharma and Delsam Pharma, both manufacturers of OTC preparations in India. The brand most implicated was sold by drug stores in the U.S as EzriCare Artificial Tears. According to the Centers for Disease Control and Prevention, there have been 68 cases of ophthalmitis of varying severity in sixteen states. The outbreak required 16 hospitalizations with eight afflicted by blindness, four involving enucleations and three fatalities.
EGG-NEWS has reported previously on eye infections arising from the use of contaminated eye drops manufactured by Global Pharma and Delsam Pharma, both manufacturers of OTC preparations in India. The brand most implicated was sold by drug stores in the U.S as EzriCare Artificial Tears. According to the Centers for Disease Control and Prevention, there have been 68 cases of ophthalmitis of varying severity in sixteen states. The outbreak required 16 hospitalizations with eight afflicted by blindness, four involving enucleations and three fatalities.
The pathogen responsible for the outbreak is an extensively drug-resistant strain of Pseudomonas aeruginosa. This strain, prevalent in India and previously unknown in the U. S., is carbapenem-resistant and carries genes expressing extended spectrum β-lactamase and metallo-β-lactamase.
The Centers for Disease Control and Prevention has issued warnings and the Food and Drug Administration has recalled both EzriCare and Delsam Pharma products.
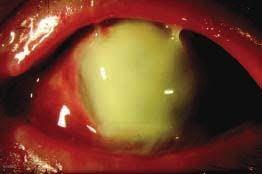
According to Dr. Marissa Grossman, a CDC Epidemic Intelligence Service Officer, the pathogen can be carried asymptomatically and screening is now underway in a number of healthcare facilities. There is evidence that the infection may be transmitted from patients to unaffected contacts. Introduction of a Pseudomonas carrying VIN and GES genes is a potentially serious health hazard.
It is questioned whether FDA has inspected the pharmaceutical plants producing the contaminated products and whether further surveillance will be instituted as a result of the outbreak. Once again EGG-NEWS questions why the U.S has to import eye drops from India or for that matter any other nation. We must re-shore manufacture of pharmaceuticals including OTCs and generics.